Continuum Model of Retinal Waves in Starburst Amacrine Cells
Filed under:
Computational neuroscience
Benjamin Lansdell (Department of Applied Mathematics, University of Washington), J. Nathan Kutz (Department of Applied Mathematics, University of Washington)
Retinal waves are an example of spontaneous activation in the developing central nervous system and are thought to play a role in retinotopic development. This activity occurs in developing neural circuits prior to any visual stimulus. The waves are the result of neighboring retinal cells spiking in a coordinated fashion which spreads over the retina. Computational models of retinal waves are used to test mechanisms of wave generation and can also be used to highlight mechanism-independent features of the waves. Elucidating the role these wave structures play in retinotopic development requires a precise mechanistic understanding to allow for insightful experimental manipulation. In rodents the most characterized waves exist in a network of cholinergic starburst amacrine cells (SACs). [1]
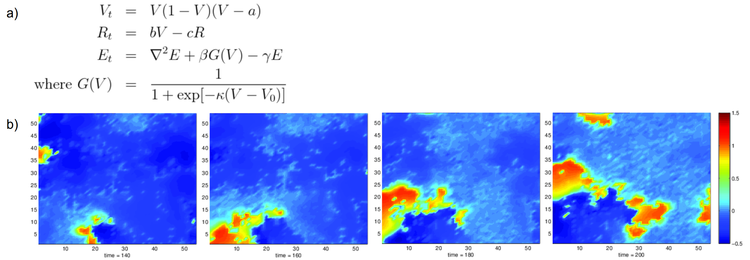
We develop a continuous spatial and temporal model of these waves in order to understand how their structure depends on underlying parameters. We use a Fitzhugh-Nagumo model of neuron dynamics and, following the study by Ford et al. [2], include spatial coupling via the diffusion of neurotransmitter – here acetylcholine (Figure a). Our simplified model allows us to study retinal waves as a reaction-diffusion type system whose role in pattern formation in biological systems is well documented. Preliminary results show that our model is able to produce qualitatively the key features of recorded waves. Similar to [2] our model suggests that cell to cell variability is a necessary component of the system needed to generate realistic localised wave structures (Figure b).
Future work includes determining how the speed and size of waves depend on the model parameters -- afforded by the use of a reaction-diffusion type model -- and investigating the role noise plays in the wave structures formed. Retinal waves are one, well-studied, example of patterned spontaneous activity in the developing central nervous system therefore our efforts represent a novel approach to studying the self-organization of neural activity more generally.
[1] Aaron G Blankenship and Marla B Feller. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nature reviews. Neuroscience, 11(1):18–29, 2010. http://dx.doi.org/10.1038/nrn2759
[2] Kevin J Ford, Aude L Felix, and Marla B Feller. Cellular mechanisms underlying spatio-temporal features of cholinergic retinal waves. Journal of neuroscience, 32(3):850–63, 2012. http://dx.doi.org/10.1523/JNEUROSCI.5309-12.2012
We develop a continuous spatial and temporal model of these waves in order to understand how their structure depends on underlying parameters. We use a Fitzhugh-Nagumo model of neuron dynamics and, following the study by Ford et al. [2], include spatial coupling via the diffusion of neurotransmitter – here acetylcholine (Figure a). Our simplified model allows us to study retinal waves as a reaction-diffusion type system whose role in pattern formation in biological systems is well documented. Preliminary results show that our model is able to produce qualitatively the key features of recorded waves. Similar to [2] our model suggests that cell to cell variability is a necessary component of the system needed to generate realistic localised wave structures (Figure b).
Future work includes determining how the speed and size of waves depend on the model parameters -- afforded by the use of a reaction-diffusion type model -- and investigating the role noise plays in the wave structures formed. Retinal waves are one, well-studied, example of patterned spontaneous activity in the developing central nervous system therefore our efforts represent a novel approach to studying the self-organization of neural activity more generally.
[1] Aaron G Blankenship and Marla B Feller. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nature reviews. Neuroscience, 11(1):18–29, 2010. http://dx.doi.org/10.1038/nrn2759
[2] Kevin J Ford, Aude L Felix, and Marla B Feller. Cellular mechanisms underlying spatio-temporal features of cholinergic retinal waves. Journal of neuroscience, 32(3):850–63, 2012. http://dx.doi.org/10.1523/JNEUROSCI.5309-12.2012
Preferred presentation format:
Poster
Topic:
Computational neuroscience

 Latest news for Neuroinformatics 2011
Latest news for Neuroinformatics 2011 Follow INCF on Twitter
Follow INCF on Twitter