Information Transfer and Recovery in the Somatosensory Cortex
Filed under:
Electrophysiology
Chao Huang (Laboratory of Neural Circuits and Plasticity, University of Southern California), Andrey Resnik (Laboratory of Neural Circuits and Plasticity, University of Southern California), Tansu Celikel (Laboratory of Neural Circuits and Plasticity, University of Southern California)
Information processing in the brain requires signal transformation every time information is transferred from one neuron to another. This transformation is performed by the postsynaptic neuron by integrating spatiotemporally distributed synaptic inputs and generating action potentials to inform its own postsynaptic partners. During this transformation how much information is retained, how much of it is transferred to postsynaptic neurons and how does the network reconstruct the lost information are unknown. Here we addressed these questions using Shannon information theory on simultaneously studied synaptic inputs, postsynaptic membrane potentials and action potentials, and network simulations.
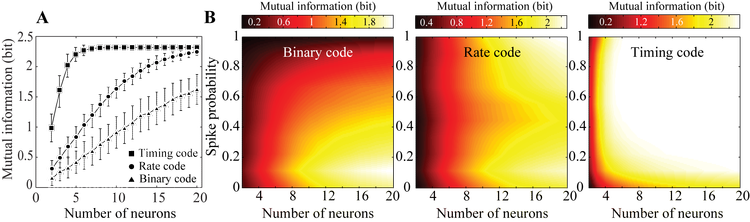
Whole cell current clamp recordings were performed in acute slices to study evoked responses in layer (L) 2/3 pyramidal neurons of the mouse primary somatosensory cortex. Electrical presynaptic stimulation, mimicking L4 responses studied during principal and surround whisker deflections in vivo, were delivered using a bipolar electrode in L4. Results show that while postsynaptic membrane potentials contain significant information about stimulus, roughly equivalent to the stimulus entropy, when subthreshold information is converted to spikes there is significant information loss (1.86±0.17 vs. 0.36±0.14 bit, p<0.001). This loss cannot be explained by the membrane state. Mutual information calculations between stimulus and spikes in simulations on the reconstructed barrel cortical column showed that although spikes from single L2/3 neurons are not information rich (0.28±0.15 bit) all the information about the stimulus can be successfully recovered by integrating responses from a pool of 8-20 local L2/3 neurons. The variance was explained by the different information encoding mechanisms that the network can hypothetically utilize; while the information in spike timing was significantly more than that of in firing-rate or the information in binary responses, minimum number of neurons required to reconstruct the lost information was significantly smaller if spike timing were used as the encoding mechanism (Figure 1).
These results show that although significant amount of information is lost when a neuron converts information it receives into spikes, local networks are able to overcome the information loss by integrating residual information across a small number of neurons. These findings argue against the notion that sensory representations can be reconstructed from single cell responses and suggest that encoding information through spike timing is the most optimal solution for neural representation of the sensory information.
Whole cell current clamp recordings were performed in acute slices to study evoked responses in layer (L) 2/3 pyramidal neurons of the mouse primary somatosensory cortex. Electrical presynaptic stimulation, mimicking L4 responses studied during principal and surround whisker deflections in vivo, were delivered using a bipolar electrode in L4. Results show that while postsynaptic membrane potentials contain significant information about stimulus, roughly equivalent to the stimulus entropy, when subthreshold information is converted to spikes there is significant information loss (1.86±0.17 vs. 0.36±0.14 bit, p<0.001). This loss cannot be explained by the membrane state. Mutual information calculations between stimulus and spikes in simulations on the reconstructed barrel cortical column showed that although spikes from single L2/3 neurons are not information rich (0.28±0.15 bit) all the information about the stimulus can be successfully recovered by integrating responses from a pool of 8-20 local L2/3 neurons. The variance was explained by the different information encoding mechanisms that the network can hypothetically utilize; while the information in spike timing was significantly more than that of in firing-rate or the information in binary responses, minimum number of neurons required to reconstruct the lost information was significantly smaller if spike timing were used as the encoding mechanism (Figure 1).
These results show that although significant amount of information is lost when a neuron converts information it receives into spikes, local networks are able to overcome the information loss by integrating residual information across a small number of neurons. These findings argue against the notion that sensory representations can be reconstructed from single cell responses and suggest that encoding information through spike timing is the most optimal solution for neural representation of the sensory information.
Preferred presentation format:
Poster
Topic:
Electrophysiology

 Latest news for Neuroinformatics 2011
Latest news for Neuroinformatics 2011 Follow INCF on Twitter
Follow INCF on Twitter