Reciprocal inhibition and slow calcium decay in perigeniculate interneurons explain changes of spontaneous firing of thalamic cells caused by cortical inactivation.
Filed under:
Computational neuroscience
Jacek Rogala (Dept. of Neurophysiology, Nencki Institute, 02-093 Warsaw), Wioletta Waleszczyk (Dept. of Neurophysiology, Nencki Institute, 02-093 Warsaw), Szymon Łęski (Dept. of Neurophysiology, Nencki Institute, 02-093 Warsaw), Andrzej Wróbel (Dept. of Neurophysiology, Nencki Institute, 02-093 Warsaw), Daniel Krzysztof Wójcik (Dept. of Neurophysiology, Nencki Institute, 02-093 Warsaw)
The role of cortical feedback in thalamocortical processing loop has been extensively investigated over the last decades. With exception of several cases, these searches focused on cortical feedback
exerted onto thalamo-cortical relay (TCR) cells of the dorsal lateral geniculate nucleus (LGN). In a
previous, physiological study we showed in the cat visual system that cessation of cortical input,
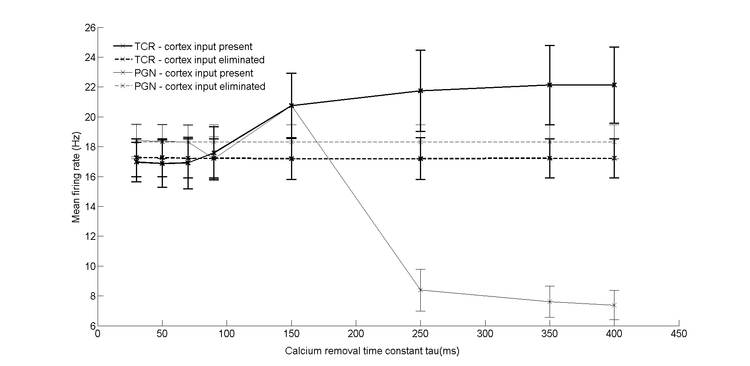
despite decrease of spontaneous activity of TCR cells, increased spontaneous firing of their recurrent inhibitory interneurons located in the perigeniculate neucleus (PGN).
To identify mechanisms underlying such functional changes we conducted a modeling study in
NEURON on several networks of point neurons with varied model parameters, such as membrane
properties, synaptic weights and axonal delays. We considered six network topologies of the retino-
geniculo-cortical system. All models were robust against changes of axonal delays except for the delay between LGN feed-forward interneuron and TCR cell. Models were manually tuned to achieve results closest to the experimental ones and than conformance of the models' output was verified by systematic search in the parameter space . The best representation of physiological results was obtained with models containing reciprocally connected PGN cells driven by the cortex assuming relatively slow decay of intracellular calcium. This strongly indicates that the thalamic reticular nucleus plays an essential role in the cortical influence over thalamo-cortical relay cells while the thalamic feed-forward interneurons are not essential in this process. Further, we suggest that the dependence of the activity of PGN cells on the rate of calcium removal can be one of the key factors determining individual cell response to elimination of cortical input.
exerted onto thalamo-cortical relay (TCR) cells of the dorsal lateral geniculate nucleus (LGN). In a
previous, physiological study we showed in the cat visual system that cessation of cortical input,
despite decrease of spontaneous activity of TCR cells, increased spontaneous firing of their recurrent inhibitory interneurons located in the perigeniculate neucleus (PGN).
To identify mechanisms underlying such functional changes we conducted a modeling study in
NEURON on several networks of point neurons with varied model parameters, such as membrane
properties, synaptic weights and axonal delays. We considered six network topologies of the retino-
geniculo-cortical system. All models were robust against changes of axonal delays except for the delay between LGN feed-forward interneuron and TCR cell. Models were manually tuned to achieve results closest to the experimental ones and than conformance of the models' output was verified by systematic search in the parameter space . The best representation of physiological results was obtained with models containing reciprocally connected PGN cells driven by the cortex assuming relatively slow decay of intracellular calcium. This strongly indicates that the thalamic reticular nucleus plays an essential role in the cortical influence over thalamo-cortical relay cells while the thalamic feed-forward interneurons are not essential in this process. Further, we suggest that the dependence of the activity of PGN cells on the rate of calcium removal can be one of the key factors determining individual cell response to elimination of cortical input.
Preferred presentation format:
Poster
Topic:
Computational neuroscience

 Latest news for Neuroinformatics 2011
Latest news for Neuroinformatics 2011 Follow INCF on Twitter
Follow INCF on Twitter